Multidrug-resistant Tuberculosis (MDR- TB)
INTRODUCTION
MDR-TB is a form of TB caused by mycobacteria that have become resistant to the two most effective anti-TB drugs, isoniazid and rifampicin. As a consequence, MDR-TB needs to be treated with other drugs, known as second-line drugs. The treatment is longer, more expensive and more difficult. Like other bacteria, mycobacteria can undergo genetic changes (known as mutations), which can make them naturally resistant to an anti-TB drug. In this instance, treatment with 3 or 4 drugs can prevent the resistant mycobacteria from growing and replacing ‘sensitive’ mycobacteria that have been eliminated by the usual TB treatment.
If resistant mycobacteria develop further resistance to another drug, the entire mycobacterial
population may be replaced with mycobacteria that have mutated at least twice and have become resistant to two drugs. Resistant mycobacteria can be transmitted to other people, who will be resistant to the usual TB treatment straight away.
CAUSES
MDR-TB always occurs as a result of human error, for example when someone does not finish their full course of treatment. The main causes of MDR-TB are:
• Inappropriate medical prescription
• Poor quality of anti-TB drugs
• Interruptions in treatment
• Nonexistent national TB control programmes
• Lack of standardised guidelines
• Inefficient supervision by healthcare providers
• Failure to complete treatment.
Failure to suspect or detect MDR-TB will give resistant mycobacteria more time to spread to other individuals in the community and worsen the problem.
SYMPTOMS
MDR-TB causes identical symptoms and involves the same organs as usual TB (weight loss,
low-grade fever and tiredness, with cough, sputum production and chest pain if mycobacteria
are in the lungs) but the disease will last longer because the mycobacteria are removed more
slowly or are not destroyed at all.
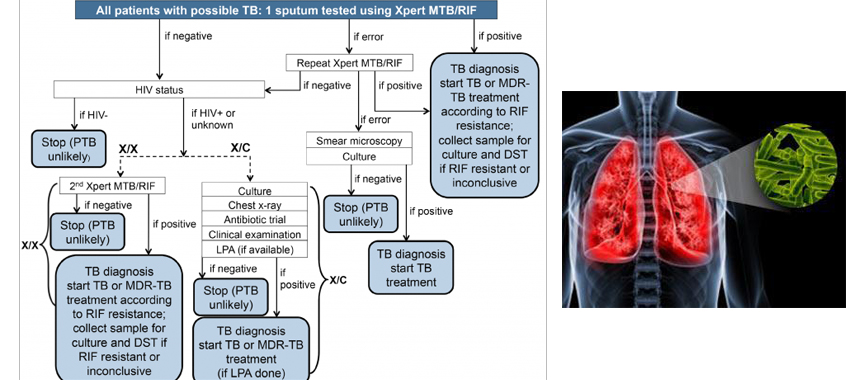
DIAGNOSIS
Drug-resistant TB often goes undetected and untreated in many countries. With the exception of a few developed countries, most national TB programs worldwide do not routinely provide diagnostic services based on culture and DST. The laboratory is an essential component in TB control programs, and broader access to DST is a priority for most countries. Early choice of appropriate treatment is an essential determinant of favourable outcome, and rapid determination of drug resistance can allow a customized approach to treatment early in the course of the disease and can potentially reduce morbidity, mortality and infectiousness
Using standardized DST procedures with conventional methods, eight to 12 weeks are required to identify drug-resistant microorganisms on solid media (ie, Lowenstein-Jensen medium).
Automated liquid culture systems are more sensitive than solid media cultures, and they significantly reduce turnaround time. However, even with liquid cultures, two to four weeks are still needed to obtain results, and their substantially higher cost is an issue for resource-limited countries. The BACTEC 460 TB radiometric system (Becton Dickinson, USA) was considered to be a major advancement when it was introduced, but has been replaced by the Mycobacteria Growth Indicator Tube system (Becton Dickinson, USA).
The identification of specific mutations responsible for drug resistance has facilitated the development of novel, rapid molecular tools for DST. The detection of RIF resistance is traditionally used as a predictor of MDR-TB – its positive predictive value is a function of the sensitivity and specificity of RIF resistance testing and the prevalence of MDR and non-MDR RIF resistance, which is highest among previously treated cases in settings with high MDR prevalence and low non-MDR RIF resistance.
Commercially available line probe assays include the INNO-LiPA Rif. TB kit (Innogenetics, Belgium) and the GenoType MTBDR assay (Hain Lifescience, Germany), Gene Xpert (Best one available)
TREATMENT
The recommended treatment combines all first-line drugs to which the strain is still sensitive, an injectable drug and one of several second-line drugs, including quinolones, prothionamide/ethionamide, cycloserine and linezolid. Treatment can last up to 2 years and is often accompanied by more or less severe side-effects. MDR-TB can represent a huge financial burden to national TB programmes, costing 10 to 100 times as much as usual TB treatment. This may put the management of other patients in danger if resources are limited.
PREVENTION
1) Treat the source of the problem. Prescribing the correct drugs from the beginning and
offering supervised treatment will increase the chance of cure and reduce the risk of symptoms
returning.
2) Detect and treat MDR-TB patients with appropriate drug combinations. This is achieved with
drug sensitivity tests using second-line drugs in an appropriate combination, and managing
side-effects and supporting the patient until they are cured.
3) Prevent the transmission of MDR-TB to other individuals by keeping the patient in isolation
until they no longer show symptoms.
References:
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2605858/
http://www.uptodate.com/contents/diagnosis-treatment-and-prevention-of-drug-resistant-tuberculosis
http://www.who.int/tb/challenges/mdr/en/
http://en.wikipedia.org/wiki/Multi-drug-resistant_tuberculosis







